-40%
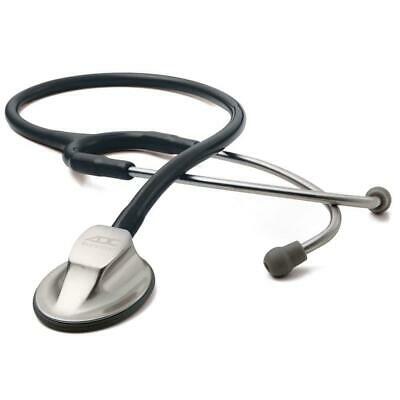
CONTEC Internal medicine hospital stethoscope Heart pulse stethoscope Amplify
$ 4.74
- Description
- Size Guide
Description
CONTEC Internal medicine hospital stethoscope Heart pulse stethoscope Amplify1.
Intended use and characteristic
Intended use:
it is
used to collect and amplify the sound
inside body organs like
heart, lung, arteries, vein
, etc
.
, which
is applicable for use in hospital, clinic, examination institution, ambulance, home and
camping rescue, etc.
Contraindications: no
Characteristic
s
:
100
Hz
~
500
Hz
as the reference of the testing sound source:
Outer diameter of auscultation head
≤
35
mm
, damping
≤
30
dB
Outer diameter of auscultation head >
35
mm
, damping
≤
16
dB
500
Hz
~
1000
Hz
as the reference of the testing sound source:
Outer diameter of auscultation head
≤
35
mm
, damping
≤
25
dB
Outer diameter of auscultation head >
35
mm
, damping
≤
20
dB
2.
Structure
It is composed of auscultation head,
hose
and
earring.
3. Use method
1)
Open the packaging to take out the device, check whether the connection among each component is well.
2)
Adjust the elasticity of the spring sheet to a comfortable position according to the distance of ears, pull the earring to both sides to loosen them (as Figure 1), otherwise to tighten them (as Figure 2).
3)
Wea
r earring and earplugs: before wearing the earring, stretch the earring outward (as Figure 3), the metal auscultation hose should be worn leaning forward, when placing the earplugs to the external auditory canals, as the shape of ears and the size of ear canals for everyone are different, the earplugs and external auditory canals should fit closely (as Figure 4), otherwise the comfort and effect of auscultation will be affected. The wrong auscultation posture is not good for auscultation, such as wearing the earring in th
e reverse direction
(as Figure 5)
.
4)
Tap the diaphragm to hear the effe
ct of sound.
Single use stethoscope can auscultate directly
4.
Precaution
1)
Don't
place
the device in environment with
high temperature, low temperature, chemical solvents and oils.
.
2)
Do not expose the device into the sunlight directly for a long time.
3)
Do not immerse the device into any liquid, and high temperature
disinfection is not permitted.
4)
When using, please don
’
t beat the device hard to avoid damaging the diaphragm (replace the accessory in time if damaged).
5)
Handle with care, do not fall, to avoid any damage to the device, affects the auscultation effect and appearance.
6)
Do not disassemble or assemble the rotating shaft by yourself, to avoid affecting the accuracy.
7)
Please clean the dirt in the earplugs periodicall
y to
avoid affecting
t
he auscultation effect.
8)
If the accident occurs during using, please call the emergency hotline immediately and seek help from the medical professional.
5.
Others
Replacement of diaphragm: unscrew the clamping ring on the auscultation head to take out the old diaphragm, then place the new one into the clamping ring, after installing the clamping ring properly, please ensure that there is no obvious looseness for the diaphragm.
Replacement of earplugs: unplug or unscrew the old earplugs, insert or rotate the new one properly.
Maintenance method: use
a soft cloth dipped alcohol to disinfect.
Transport and storage environment:
Temperature: -
40
℃
~
+
55
℃
Relative humidity: 10
%
~
80
%
Service life: 5 years
Date of manufacture: see the packaging
*
The actual service life depends on daily use frequency, storage environment (temperature, humidity, etc.) and storage method, please use and store it reasonably.
Warning: the disposal of the device, components and optional accessories should follow the local laws and regulations, as improper disposal may pollute the environment
Payment Way !!
* We accept payment from PAYPAL, Bank
transfer,Visa,Credit Card and others.
* All payments are expected within 7 days after the
last winning auction is closed.
Buy Safe Product!!
■
The following FDA Disclaimer is required for all eBay listing in
Healthcare category and is included for REFERENCE:
■
The sale of this item may be subject to regulation by the U.S.
Food and Drug Administration and state and local regulatory agencies.
■
If the item is subject to FDA regulation,We will verify your status
as an authorized purchaser of this item before shipping of the item.
■
The
model
is registered on the Australian Register of Therapeutic
Goods (ARTG)
with the code 197923,
and certified by FDA
510K Approved of America and CE,TUV of Europe
About Us
Contec Medical Systems Co., Ltd. (hereinafter referred to as CONTEC) focusing on research, manufacture and distribution of medical instruments, was founded in 1996 as a high-tech company. CONTEC locates in Economic & Technical Development Zone in Qinhuangdao covered an area of 125 acres and building area of over 100000 square meter, which is one of the largest bases for R & D and production of medical devices in China.
Contec hopes to cooperate with international companies to supply more innovative design and advanced
technology products
We sincerely welcome you to become one of our global partners.
We are looking forward to establishing a successful business relationship with you.
Powered by SoldEazy
Only English user guide,if you need any other language,please contact us!